A new mechanism in the formation of ribosomes has been discovered by
researchers from the Heidelberg University Biochemistry Center. In an
interdisciplinary approach, the Heidelberg scientists, along with
colleagues from Switzerland and Japan, describe a heretofore
uncharacterised protein that plays a specific role in ribosome assembly
in eukaryotes, organisms whose cells contain a cell nucleus. This
protein makes sure that specific factors required for ribosome synthesis
are transported together, like hitchhikers, into the nucleus to the
site of assembly. The results of this research were published in
“Science”.
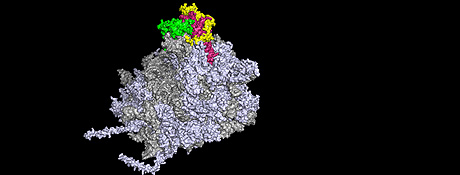
Ribosomes, the protein factories of the cell, are macromolecular complexes of ribonucleic acids (RNA) and ribosomal proteins (r-proteins) that are organised in a highly complicated three-dimensional nanostructure. Correct synthesis of ribosomes is critical for the division of all cells and is a process that follows strict rules. In eukaryotes, new ribosomes are formed predominantly in the cell nucleus. Therefore, the r-proteins needed for ribosome formation must travel from the cytoplasm of the cell to a site in the nucleus where the ribosomes are assembled. Until recently it was not clear whether r-proteins that have a similar function and form functional clusters on the ribosome structure are also co-transported into the nucleus.
The researchers have now found a protein that coordinates the co-transport of certain r-proteins in functional clusters into the cell nucleus. This factor is called Symportin1, for synchronised import. “Symportin1 synchronises the import of both the Rpl5 and Rpl11 r-proteins into the cell nucleus and supports their integration into the growing ribosome structure”, explains Prof. Dr. Irmgard Sinning of the Heidelberg University Biochemistry Center (BZH). “It employs a familiar logistical concept from every day life, like picking up a hitchhiker or sharing a taxi with someone headed for the same destination”, says Dr. Gert Bange of the BZH, lead author of the study together with Dr. Dieter Kressler (now of Fribourg University).
source: http://www.uni-heidelberg.de/presse/news2012/pm201211102_ribosomen_en.html
Ribosomes, the protein factories of the cell, are macromolecular complexes of ribonucleic acids (RNA) and ribosomal proteins (r-proteins) that are organised in a highly complicated three-dimensional nanostructure. Correct synthesis of ribosomes is critical for the division of all cells and is a process that follows strict rules. In eukaryotes, new ribosomes are formed predominantly in the cell nucleus. Therefore, the r-proteins needed for ribosome formation must travel from the cytoplasm of the cell to a site in the nucleus where the ribosomes are assembled. Until recently it was not clear whether r-proteins that have a similar function and form functional clusters on the ribosome structure are also co-transported into the nucleus.
The researchers have now found a protein that coordinates the co-transport of certain r-proteins in functional clusters into the cell nucleus. This factor is called Symportin1, for synchronised import. “Symportin1 synchronises the import of both the Rpl5 and Rpl11 r-proteins into the cell nucleus and supports their integration into the growing ribosome structure”, explains Prof. Dr. Irmgard Sinning of the Heidelberg University Biochemistry Center (BZH). “It employs a familiar logistical concept from every day life, like picking up a hitchhiker or sharing a taxi with someone headed for the same destination”, says Dr. Gert Bange of the BZH, lead author of the study together with Dr. Dieter Kressler (now of Fribourg University).
source: http://www.uni-heidelberg.de/presse/news2012/pm201211102_ribosomen_en.html